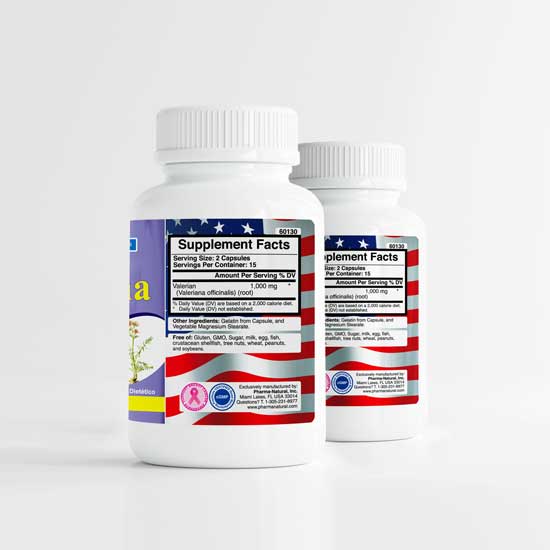
Valerian Root
Valerian Root by PN -2 (30 caps), A Natural Sleep & Anxiety Management Aid
by PN -2 (30 caps), A Natural Sleep & Anxiety Management Aid
$8.40
These statements have not been evaluated by the Food and Drug Administration (FDA). This product has not been evaluated by the FDA. It is not intended to diagnose, treat, cure or prevent any disease.